CWENCH Hydration™ has become the title sponsor of the CWENCH All Canadian Basketball Games, alongside other sponsors including Nike, TSN and Team Town. Cizzle Brands continues to build out its portfolio of sponsorships in Canadian grassroots sports, driving visibility for CWENCH Hydration™ as a healthy and great-tasting hydration option for athletes of all ages.

Cleo Diagnostics Investor Kit
- Corporate info
- Insights
- Growth strategies
- Upcoming projects
GET YOUR FREE INVESTOR KIT
Cleo Diagnostics
Overview
A medical technology company based in Australia, Cleo Diagnostics (ASX:COV) is revolutionising women's healthcare with its disruptive cancer detection platform technology, through a simple blood test that can accurately detect ovarian cancer early – the leading cause of cancer-related deaths among women.
Approximately 50 percent of women will die within five years of an ovarian cancer diagnosis. The chances of survival beyond five years, however, increase with early detection. According to the American Cancer Society, only about 20 percent of ovarian cancers are diagnosed at an early stage, and more than 90 percent of women live beyond five years when the cancer is detected early.

With early diagnosis being key to a higher survival rate, ovarian cancer has become a target for biomarker research. And one particular biomarker holds promise.
Cleo’s technology is underpinned by the CXCL10 novel and patented biomarker, which was first identified as a small inflammatory molecule in ovarian cancer tissue sections. Subsequent research demonstrated that CXCL10 was overexpressed in ovarian cancers, but importantly not expressed in benign disease, and remains throughout the lifetime of the cancer. The biomarker effectively provides a robust indicator at all stages of cancer. Recognizing that early detection is a significantly unmet need in the clinical diagnostics market, Cleo Diagnostics is focused on bringing to market a simple blood test to accurately detect ovarian cancer early.
Cleo’s first clinical validation study for its ovarian cancer triage test has been published in the peer-reviewed international journal Cancers. The article concluded that Cleo’s ovarian cancer test was highly accurate with 95 percent sensitivity and 95 percent specificity, correctly discriminated malignant from benign samples, and has outperformed and was superior to current clinical methods. The second peer-reviewed dataset has also been published in the medical journal Diagnostics, which concluded that CLEO’s test has correctly identified most cancer cases that were missed by the standard marker CA125. It also eliminated the majority of “false positive” results caused by CA125 use, and it correctly identified the majority of patients with early-stage ovarian cancers.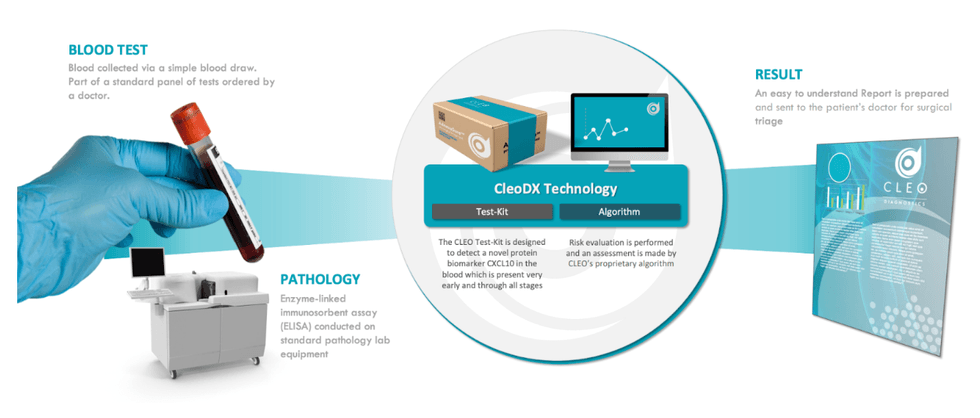
CLEO has appointed New York-based healthcare industry consultancy, HcFocus, to support the commencement of its US market access program. HcFocus will provide specialised and strategic expertise to assist CLEO in navigating the complexities of the US health system and regulatory environment.
The addressable market for a technology like this is compelling, and with a management team that brings to the table decades of leadership experience in the medical technology space, Cleo is well-positioned to leverage this market opportunity.
Cleo chief executive and executive director Dr. Richard Allman has over 30 years of experience in commercially focused scientific research and innovation. Throughout his career, Allman has overseen and expedited a product development pipeline covering no less than six major cancers, cardiovascular disease, type-2 diabetes and a commercially available COVID-19 test.
Chief scientific officer Dr. Andrew Stephens boasts an equally impressive resume. A career research scientist with two decades of experience in molecular and cellular biology, Stephens is named in over 60 academic publications and holds numerous patents in the cancer therapy and diagnostic space. Cleo’s blood test looks for a novel and patented biomarker in the blood called CXCL10, which was discovered by Stephens, the product of over ten years of scientific work at Monash Medical Centre's Hudson Institute of Medical Research.
There's also Professor Tom Jobling, Cleo's non-executive director and lead medical advisor. As the head of gynaecological oncology at Monash Health and visiting medical officer at the Peter MacCallum Cancer Centre, Jobling has been treating ovarian cancer for over thirty years. He was also the founding chairman of the Ovarian Cancer Research Foundation (OCRF)
Non-executive director Lucinda Nolan, meanwhile, brings significant business and strategic expertise to the table. Most recently, she served as the CEO of the Ovarian Cancer Research Foundation.
These experienced professionals, together with the other members of Cleo’s management and board, have developed a staged execution strategy focused on de-risking the pathway to the international screening market — ensuring that, although Cleo is still in its advanced R&D stage, its prospects for commercialisation remain incredibly promising.
Company Highlights
- Backed by medical professionals and cancer specialists with decades of experience, Cleo Diagnostics has developed a disruptive, accurate and early-stage ovarian cancer detection blood test.
- Cleo targets the CXCL10 novel biomarker, which is now known to be overexpressed in all stages of ovarian cancer.
- Cleo is the result of more than a decade of research at the Hudson Institute of Medical Research, where chief scientist Dr. Andrew Stephens received more than $5 million OCRF & NHMRC funding for development and clinical studies.
- The test is also supported by Professor Tom Jobling, founder of the Ovarian Cancer Foundation and Lucinda Nolan, the foundation's former CEO.
- Cleo has developed a staged execution strategy focused on an achievable path to market. This ensures the project, which is currently in its advanced R&D stage, can maximise commercial value for all stakeholders.
Key Projects
Cleo Diagnostics
Developed over a decade by Dr. Andrew Stephens, Cleo’s blood test is underpinned by the CXCL10 novel and patented protein biomarker known to be present in all stages of ovarian cancer. By combining CXCL10 with several other biomarkers in a custom algorithm, Cleo can not only be used in triage, but also for screening and recurrence testing. The project is currently in the advanced R&D stage and has so far conducted two clinical studies, analysing more than 700 patient samples in the process.
Highlights:
- Readily Accessible: Cleo requires no additional or specialised equipment and can be conducted in any standard pathology lab either on its own or as part of a standard panel of tests ordered by a physician.
- AI-based Risk Assessment: Once the sample has been collected and tested, Cleo leverages a proprietary algorithm to perform a risk evaluation on the patient, determining the likelihood of a cancer diagnosis.
- Intuitive Results: Cleo generates an easy-to-understand post-assessment report which can then be sent to the patient's primary care provider or surgeon for triage.
- High Performance: The Cleo prototype outperforms FDA-cleared predicates and clinical guideline tests in terms of accuracy and specificity.
- Current Roadmap: Cleo plans for the test to be ready for clinical use in a surgical triage setting by 2025, where it will be available initially to one million patients. Target launch dates for recurrence, high-risk screening and mass screening are still to be determined. Additionally, the company has numerous inflection points planned over the next two years:
- Kit Development:
- Internal trial antibody optimisation
- Finalisation of antibody selection for the Cleo test-kit
- Complete re-agent development
- Pre-IDE strategic development
- Manufacturing:
- Establishment and accreditation of ISO13485 quality system
- Manufacturing establishment of Cleo key biomarker
- Manufacturing establishment of Cleo Ovarian Cancer Kit
- Clinical Studies:
- Sign key opinion leaders and trial sites
- Perform and finalise verification of the Cleo kit through clinical studies
- Regulatory Approval:
- FDA Pre-IDE submission
- CE regulatory submissions and approval
- TGA regulatory submission and approval
- FDA submission and approval
- Kit Development:

Cleo is bringing to market three testsfor ovarian cancer diagnosis, monitoring and screening.
Management Team
Dr. Richard Allman — Chief Executive Officer and Executive Director
Dr. Richard Allman has over 30 years of scientific research leadership and innovation with a clear focus on commercialisation. He has wide experience in research leadership, innovation management, and intellectual property strategy, covering oncology, diagnostics, and product development.
Previously, Allman was chief scientific officer at Genetic Technologies (ASX:GTG). Recent successes include the strategic design and management of a second-generation breast cancer risk assessment test from concept to commercial launch and a similar test for colorectal cancer. These tests have now been NATA-accredited and comprise the first commercially available polygenic risk tests in Australia.
More recently, Allman supervised the underlying R&D, translation, regulatory approval, patent filing and commercial launch of a COVID-19 disease severity test within 12 months. This strategy has been utilised to expedite a product development pipeline covering six major cancers, cardiovascular disease and type-2 diabetes which were commercially launched in March 2022.
Dr. Andrew Stephens — Chief Scientific Officer and Executive Director
Dr. Andrew Stephens is a career research scientist with 20 years of experience in molecular and cellular biology research. He has broad experience in academic and pre-clinical research and a strong focus on translation and the commercialisation of research findings. He established and leads an independent academic research group at the Hudson Institute of Medical Research, investigating mechanisms that contribute to the formation, progression and dissemination of high-grade, serous epithelial ovarian cancers. Since 2010, his research has focused on biomarker identification and development in ovarian cancer and the development of therapeutic strategies to improve patient outcomes. He is also actively involved across the biotech sector, with appointments to the scientific advisory for Invion and AMTBio.
Stephens has more than 60 academic publications and numerous patents (pending and provisional) in the cancer therapeutic and diagnostic space.
Professor Tom Jobling — Lead Medical Advisor and Non-executive Director
Professor Thomas Jobling is director of gynaecologic oncology at Monash Medical Centre. He graduated from Monash University in 1980 and did his postgraduate sub-specialist training in gynaecologic oncology in London at the Royal Marsden and St Bartholomew's hospitals. Jobling has subsequently been elected as a member of the Society of Pelvic Surgeons and is also founder of the Ovarian Cancer Research Foundation (1999). He was the chairman of the Ovarian Cancer Research Foundation Board. His major interests are in radical surgery for ovarian cancer and the application of robotic surgery for gynaecological malignancy.
Jobling is an active member of a research team in biomarker detection and proteomics in ovarian cancer. He is involved as a collaborative investigator on a number of international clinical trials and is a member of the Australia and New Zealand Gynaecologic Oncology Group, the Australian Society of Gynaecologic Oncology, the Victorian Cooperative Oncology Group and the International Society of Gynaecological Cancer.
Lucinda Nolan — Non-executive Director
Lucinda Nolan is a non-executive director and was most recently the CEO of the Ovarian Cancer Research Foundation. She has a wealth of knowledge and experience across the public sector and not-for-profit environments. Before joining the Ovarian Cancer Research Foundation, she was selected as the first female CEO of the Country Fire Authority, one of the world’s largest volunteer-based emergency services organisations. She also spent 32 years with Victoria Police, reaching the rank of deputy commissioner. She was awarded the Australian Police Medal in 2009.
Nolan is also the chair of BankVic and a director on the boards of Alkira Box Hill and the Melbourne Archdiocese of Catholic Schools. She has a Master of Arts and a Bachelor of Arts (Honours) from Melbourne University and is an alum of the Advanced Management Programme at Harvard University.
Adrien Wing — Non-executive Chair
Adrien Wing began his professional career practising in the audit and corporate advisory divisions of a chartered accounting firm. He has over 25 years of experience in the corporate sector with a large portion of this experience in ASX small caps, lead in IPO transactions and post listing reverse takeovers and acquisitions across a range of industry sectors and jurisdictions. He also has a strong pedigree in the life sciences industry being the founder of Rhythm Biosciences and bringing that entity to the ASX in 2017.
Wing currently serves as an officer/director on the following company boards: New Age Exploration (ASX: NAE), director and joint company secretary; Red Sky Energy (ASX:ROG), director and joint company secretary; Sparc Technologies (ASX:SPN), company secretary; and Osmond Resources (ASX:OSM), company secretary.
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection
Quarterly activities and cashflow report
CLEO Further Expands Ovarian Cancer Trial with Siles Health
The Royal Women's Hospital Joins CLEO Ovarian Cancer Trial
Cizzle Brands Lands Title Sponsorship for the CWENCH All Canadian Basketball Games
Cizzle Brands Corporation (Cboe Canada: CZZL) (OTCQB: CZZLF) (Frankfurt: 8YF) ( the "Company" or "Cizzle Brands") , is pleased to announce that its flagship brand CWENCH Hydration™ is gaining further prominence in Canadian athletics through a five-year title sponsorship agreement of the CWENCH All Canadian Basketball Games, a three-day NBA-sanctioned event for Canadian male and female senior high school basketball players taking place on Friday, April 4, 2025 and Saturday, April 5, 2025. The event will be broadcast across Canada on TSN, and will have pre-promotion during the network's coverage of the NCAA® March Madness® tournaments this month.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250320762204/en/

CWENCH Hydration is now title sponsor of the CWENCH All Canadian Games, a three-day event that celebrates Canada's top 24 senior male and female Canadian high school basketball players.
In addition to title sponsorship of the CWENCH All Canadian Basketball Games, the five-year agreement provides Cizzle Brands with comprehensive sponsorship rights for the promotion of its brands. This includes the naming rights for the event, naming rights for the CWENCH Player of the Game, the CWENCH logo appearing on all players' jerseys, commercial spots during the national broadcast on TSN, bench rights and exclusivity in the hydration category.
Also, as part the event, all of the athletes will take part in clinical sweat-testing trials to measure the impact that CWENCH Hydration™ has on performance.
Supporting grassroots sports initiatives in Canada is core to Cizzle Brands' ethos as a company, and has proven to be especially impactful for the early-stage growth of CWENCH Hydration™ in the North American marketplace. In addition to Cizzle Brands' recently announced sponsorship of the CWENCH All Canadian Volleyball Games, CWENCH also sponsors 500 youth hockey teams in Canada, representing over 12,000 youth hockey players. Last year, Cizzle Brands also obtained a sponsorship deal with Canlan Sports for a major four-rink hockey complex in the Toronto area, which is now named CWENCH Centre - A Canlan Sports Community . These efforts have contributed to driving strong demand for CWENCH Hydration™, with many of its leading retail and distribution partners re-ordering product at a fast pace, as further detailed in the Company's March 6, 2025 press release.
The CWENCH All Canadian Games will feature the top 24 senior male and female Canadian high school basketball players as selected by a committee including provincial and national representatives, clubs, coaches, scouts, and media from across the country. At the Athlete Institute in Orangeville, Ontario (near Toronto), participants engage in a series of activities over three days. These activities include on-and-off court training, practices and scrimmages in front of NBA personnel, a 3-Point Shootout, a Slam Dunk Competition, and Boys and Girls games. As an NBA-sanctioned event, the CWENCH All Canadian Basketball Games attract representation from the majority of the National Basketball Association's organizations.
More information about the CWENCH All Canadian Basketball Games can be found on the event's website: https://www.cwenchallcanadian.com .
Cizzle Brands' Founder, Chairman, and Chief Executive Officer John Celenza commented, "We are delighted to sponsor the CWENCH All Canadian Basketball Games. The impact that this event has had on Canadians coast-to-coast is staggering. From a brand recognition standpoint, it will be instrumental to our continued growth and, as an organization, we take great pride in supporting grassroots athletics across Canada. The CWENCH All-Canadian Games will be a key catalyst for the continued growth of CWENCH Hydration™."
Jesse Tipping, CEO of the Athlete Institute Ltd., which is organizing games, added: "Cizzle Brands is an incredible Canadian company that puts the needs and health of young athletes at the forefront. I am proud to have CWENCH Hydration™ be the title sponsor for the games and look forward to our future together."
"As the Official Broadcaster of the CWENCH All Canadian Games, it's an honour for TSN to shine the spotlight on Canada's emerging young basketball players," said Shawn Redmond, VP, Bell Media Sports. "Canadian players are making a massive impact on the sport at all levels, and the CWENCH All Canadian Games are a key stepping stone, ensuring the next generation has a national platform to showcase their talents for fans."
About Cizzle Brands Corporation
Cizzle Brands Corporation is a sports nutrition company that is elevating the game in health and wellness. Through extensive collaboration and testing with leading athletes and trainers across several elite sports, Cizzle Brands has launched two leading product lines in the sports nutrition category: (i) CWENCH Hydration™, a better-for-you sports drink that is now carried in over 1,200 stores in Canada, the United States, and Europe; and (ii) Spoken Nutrition, a premium brand of athlete-grade nutraceuticals that carry the prestigious NSF Certified for Sport® qualification. All Cizzle Brands products are designed to help people achieve their best in both competitive sports and in living a healthy, vibrant, active lifestyle.
For more information about Cizzle Brands, please visit: https://www.cizzlebrands.com/
For more information about CWENCH Hydration™, please visit: https://www.cwenchhydration.com
For more information about the CWENCH All Canadian Games, please visit: https://www.cwenchallcanadian.com
Notice Regarding Images and Links: This press release may contain images and/or links to outside web pages, which could play an important role in providing the full context of the news update being conveyed through this press release. Some news aggregation services may remove these images and/or links at their discretion. Therefore, readers are encouraged to access SEDAR+ or the News section of the Cizzle Brands Corporation website to view this press release containing all images and/or links as originally published.
On behalf of the Board of Directors of the Company,
"John Celenza"
John Celenza, Founder, Chairman, and Chief Executive Officer
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This news release contains "forward-looking information" which may include, but is not limited to, information with respect to the activities, events or developments that the Company expects or anticipates will or may occur in the future, such as, but not limited to: new products of the Company and potential sales and distribution opportunities. Such forward-looking information is often, but not always, identified by the use of words and phrases such as "plans", "expects", "is expected", "budget", "scheduled", "estimates", "forecasts", "intends", "anticipates", or "believes" or variations (including negative variations) of such words and phrases, or state that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved. Various assumptions or factors are typically applied in drawing conclusions or making the forecasts or projections set out in forward-looking information. Those assumptions and factors are based on information currently available to the Company.
Forward looking information involves known and unknown risks, uncertainties and other risk factors which may cause the actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking information. Such risks include risks related to increased competition and current global financial conditions, access and supply risks, reliance on key personnel, operational risks, regulatory risks, financing, capitalization and liquidity risks. Although the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such information will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking information. The Company undertakes no obligation, except as otherwise required by law, to update these forward-looking statements if management's beliefs, estimates or opinions, or other factors change.

View source version on businesswire.com: https://www.businesswire.com/news/home/20250320762204/en/
Setti Coscarella
Head of Corporate Development
investors@cizzlebrands.com
1-844-588-2088
News Provided by Business Wire via QuoteMedia
Cizzle Brands Corporation Reports $5.64 Million in Net Sales in the First Half of its 2025 Fiscal Year
For the six-month period ended January 31, 2025, Cizzle Brands recorded over $5.64 million in net sales, with a gross profit margin of 60.03% and gross profit of over $3.38 million. These earnings were primarily driven by sales of CWENCH Hydration™ in Canada, with sustained sales of the product in the United States and Europe. The Company has publicly filed its financial results for the second quarter of its 2025 fiscal year, and its management team will be hosting a webcast to discuss these results on Thursday, March 27, 2025.
Cizzle Brands Corporation (Cboe Canada: CZZL) (OTCQB: CZZLF) (Frankfurt: 8YF) ( the "Company" or "Cizzle Brands") , today released its financial results for the second quarter of its 2025 fiscal year (three months ended January 31, 2025, referred to herein as "FQ2 2025"). The results for FQ2 2025 capped off the first half of Cizzle Brands' inaugural fiscal year as a public company, in which more than $5.64 million in net sales were generated, with 60.03% gross profit margin and gross profit of over $3.38 million.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250317771206/en/

During FQ2 2025 Cizzle Brands' common shares became listed on the Cboe Canada stock exchange, an event that was commemorated by the bell-ringing ceremony pictured above at the exchange's Toronto office
Throughout the first half of the Company's 2025 fiscal year, Cizzle Brands accelerated strategic commercialization efforts for its flagship product CWENCH Hydration™ while also carrying out the launch of Spoken Nutrition , a brand of premium, athlete-grade nutraceuticals. During this period, 71% of Cizzle Brands' sales were made in Canada, with 20% and 9% coming from the United States and Europe, respectively.
Key highlights from Cizzle Brands' FQ2 2025 earnings are listed below. All figures are in Canadian Dollars unless otherwise specified.
- Net sales for FQ2 2025 were $2,856,072 with Cost of Goods Sold being $1,216,689 for gross profit margin of 57.4%;
- Current Assets grew by 43.3% from $7,220,755 at July 31, 2024 to $10,347,341 at January 31, 2025;
- Growth of Current Assets was primarily driven by a 72.8% increase in Cash from $1,519,516 to $2,626,915 and a 126.7% increase in Inventory from $1,376,990 to $3,122,031 between the same two dates;
- All product inventory is "current" with no obsolete inventory and no provisions to inventory having been made;
- CWENCH Hydration™ is available in over 1,800 points of distribution across North America, including sites serviced by Van Houtte Coffee Services (a subsidiary of Keurig Dr Pepper), whose established Canada-wide network of over 30,000 doors reduces Cizzle Brands' overhead expenses for logistics and can allow the Company to scale the footprint of CWENCH Hydration™ more rapidly;
- Several key retail partners for CWENCH Hydration™ were added during the Company's FQ2 2025 including Metro Ontario , MacEwen Petroleum , Canco Petroleum , Fortinos , Sport Chek, Source for Sports, and Life Time Fitness , which is an upscale chain of fitness resorts with over 160 locations across North America; and
- Re-order rates for CWENCH Hydration™ have been growing steadily for the Company. In a press release dated March 6, 2025 , Cizzle Brands announced that all accounts which have existed for two or more months have placed at least one reorder. With Cizzle Brands' largest Canadian account during FQ2 2025, the two Hydration Mix versions of CWENCH Hydration™ (10-count and 315-gram) have experienced unit sales growth of 72% and 64% (respectively) between FQ2 2025 and the prior fiscal quarter.
Please refer to Cizzle Brands' profile on SEDAR+ ( http://www.sedarplus.ca/ ) to view the Company's full FQ2 2025 earnings reports, as well as its corresponding Management Discussion and Analysis ("MD&A").
Additionally, Cizzle Brands will be hosting a webcast on Thursday, March 27, 2025 at 4:30 PM ET in which the Company's management team will discuss these results in greater detail. Registration (free) is required to attend, and can be done through the following link: https://streamyard.com/watch/htXpXqNJzhUR
Please refer to the Company's March 13, 2025 press release or contact Cizzle Brands Investor Relations for more information regarding the upcoming webcast.
Cizzle Brands' Founder, Chairman, and Chief Executive Officer John Celenza commented, "The first half of our fiscal 2025 year was very successful, with the team achieving key milestones against our strategic plan. To have generated over $5.64 million in net sales in just six months is a testament to the brand strength we are creating. Our next two fiscal quarters will take place during spring and summer when sales within our hydration category tend to be higher. Based on this, Cizzle Brands is on track to surpass $14 million in net sales by the end of our first complete fiscal year."
Mr. Celenza continued, "This momentum reflects our team's dedication to growth, the quality of our products, and most importantly the traction we have begun to garner at the consumer level, which is driving repeat purchases as well as organic acquisition of new customers through word-of-mouth. We will continue scaling our business by driving growth through existing channels, and by selectively adding new channels at each step of our commercialization journey. We have significant opportunities in front of us in both Canada and the United States that we are well-positioned to capitalize on, and we will be sharing many of these developments over the coming weeks. We look forward to discussing these financial results on next week's webcast."
About Cizzle Brands Corporation
Cizzle Brands Corporation is a sports nutrition company that is elevating the game in health and wellness. Through extensive collaboration and testing with leading athletes and trainers across several elite sports, Cizzle Brands has launched two leading product lines in the sports nutrition category: (i) CWENCH Hydration™, a better-for-you sports drink that is now carried in over 1,800 stores in Canada, the United States, and Europe; and (ii) Spoken Nutrition, a premium brand of athlete-grade nutraceuticals that carry the prestigious NSF Certified for Sport® qualification. All Cizzle Brands products are designed to help people achieve their best in both competitive sports and in living a healthy, vibrant, active lifestyle.
For more information about Cizzle Brands, please visit: https://www.cizzlebrands.com/
For more information about CWENCH Hydration™, please visit: https://www.cwenchhydration.com
For more information about the CWENCH All Canadian Games, please visit: https://www.cwenchallcanadian.com
Notice Regarding Images and Links: This press release may contain images and/or links to outside web pages, which could play an important role in providing the full context of the news update being conveyed through this press release. Some news aggregation services may remove these images and/or links at their discretion. Therefore, readers are encouraged to access SEDAR+ or the News section of the Cizzle Brands Corporation website to view this press release containing all images and/or links as originally published.
On behalf of the Board of Directors of the Company,
"John Celenza"
John Celenza, Founder, Chairman, and Chief Executive Officer
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This news release contains "forward-looking information" which may include, but is not limited to, information with respect to the activities, events or developments that the Company expects or anticipates will or may occur in the future, such as, but not limited to: new products of the Company and potential sales and distribution opportunities. Such forward-looking information is often, but not always, identified by the use of words and phrases such as "plans", "expects", "is expected", "budget", "scheduled", "estimates", "forecasts", "intends", "anticipates", or "believes" or variations (including negative variations) of such words and phrases, or state that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved. Various assumptions or factors are typically applied in drawing conclusions or making the forecasts or projections set out in forward-looking information. Those assumptions and factors are based on information currently available to the Company.
Forward looking information involves known and unknown risks, uncertainties and other risk factors which may cause the actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking information. Such risks include risks related to increased competition and current global financial conditions, access and supply risks, reliance on key personnel, operational risks, regulatory risks, financing, capitalization and liquidity risks. Although the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such information will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking information. The Company undertakes no obligation, except as otherwise required by law, to update these forward-looking statements if management's beliefs, estimates or opinions, or other factors change.

View source version on businesswire.com: https://www.businesswire.com/news/home/20250317771206/en/
For further information, please contact:
Setti Coscarella
Head of Corporate Development
investors@cizzlebrands.com
1-844-588-2088
News Provided by Business Wire via QuoteMedia
New RAD202 data confirms positive tumor uptake
UPLIZNA® SIGNIFICANTLY IMPROVES GENERALIZED MYASTHENIA GRAVIS SYMPTOMS IN ACETYLCHOLINE RECEPTOR AUTOANTIBODY-POSITIVE PATIENTS OVER 52 WEEKS
Patients Reported Improvement in Ability to Conduct Daily Activities with Twice-Yearly Dosing *
Late-Breaking Data to be Presented at AAN 2025
- Amgen (NASDAQ:AMGN) today announced new data from the Phase 3, registrational MINT trial evaluating the efficacy and safety of UPLIZNA ® (inebilizumab-cdon) in adults living with generalized myasthenia gravis (gMG). The results demonstrated durable and sustained efficacy of UPLIZNA in patients with acetylcholine receptor autoantibody-positive (AChR+) gMG with two doses a year, following an initial loading dose. Findings will be presented as a late-breaking oral presentation during the American Academy of Neurology (AAN) Annual Meeting on April 8, 2025 in San Diego .
The Phase 3 MINT trial, which was a randomized-control trial, evaluated UPLIZNA in muscle-specific kinase autoantibody-positive (MuSK+) and AChR+ gMG patients, with the MuSK+ group followed for 26 weeks and the AChR+ group followed for 52 weeks. The trial demonstrated continued improvement in efficacy of UPLIZNA compared to placebo (adjusted difference, −2.8, 95% CI, −3.9 to −1.7) as measured by the change in baseline of Myasthenia Gravis Activities of Daily Living (MG-ADL) score in the AChR+ subpopulation through week 52. Among the AChR+ patients in the UPLIZNA group, 72.3% had a ≥3 point improvement in the MG-ADL score, compared to 45.2% in placebo. 1
As previously disclosed at the 2024 American Association of Neuromuscular & Electrodiagnostic Medicine Annual Meeting, the trial met its primary endpoint, with a statistically significant change from baseline in MG-ADL score for UPLIZNA (-4.2) compared with placebo (-2.2) (difference: –1.9, p
"The 52-week MINT trial results highlight the potential for a new standard of care in gMG, offering durable symptom relief with a simplified treatment regimen," said Jay Bradner , M.D., executive vice president of Research and Development at Amgen. "These findings reinforce UPLIZNA's ability to provide sustained symptom relief with just two doses per year—an important advancement for patients living with generalized myasthenia gravis—while underscoring our commitment to developing transformative therapies for people facing complex autoimmune diseases."
Change from baseline in the Quantitative Myasthenia Gravis (QMG) score was also greater for patients in the UPLIZNA group as compared to placebo at Week 52 (adjusted difference, −4.3, 95% CI, −5.9 to −2.8). Among the AChR+ patients in the UPLIZNA group, 69.2% improved by ≥3 points in the QMG score, compared to 41.8% in the placebo group. 1
MINT was the first and only Phase 3 trial for a biologic to incorporate a corticosteroid taper into its protocol. Patients who entered the study taking corticosteroids were tapered down starting at Week 4 to prednisone 5 mg per day by Week 24.
"I'm looking forward to further examining the 52-week MINT data with my colleagues in the neurology community at AAN," said Richard J. Nowak , M.D., M.S., global principal study investigator and director of the Myasthenia Gravis Clinic at Yale University . "These results showed that UPLIZNA consistently relieved burdensome symptoms and improved activities of daily living for gMG patients."
No new safety signals were identified. The overall TEAE profile during the study period is consistent with the known safety profile for the approved indication (NMOSD). The most common adverse events included infusion-related reactions, nasopharyngitis and urinary tract infections.
UPLIZNA is currently approved for the treatment of adult patients with anti-aquaporin-4 (AQP4) antibody positive neuromyelitis optica spectrum disorder (NMOSD) and is under priority FDA review for the treatment of Immunoglobulin G4-related disease (IgG4-RD) with a PDUFA date of April 3, 2025 . The FDA has granted UPLIZNA Orphan Drug Designation for the treatment of gMG. Regulatory filing activities are underway with submission anticipated to be complete in H1 2025.
*After an initial loading dose.
About the MINT Trial
The MINT trial is a randomized, double-blind, placebo-controlled, parallel-group trial ( NCT04524273 ) evaluating the efficacy and safety of UPLIZNA in adults with gMG. The trial enrolled 238 adults with gMG, including 190 patients who are acetylcholine receptor autoantibody-positive (AChR+) and 48 patients who are muscle-specific kinase autoantibody-positive (MuSK+).
Eligibility criteria at screening and randomization included a Myasthenia Gravis Foundation of America (MGFA) classification of II, III, or IV disease, MG-ADL score between 6 and 10 with greater than 50% of this score attributed to non-ocular items, or an MG-ADL score of at least 11, QMG score of at least 11, and use of a corticosteroid and/or non-steroidal immunosuppressant.
The primary endpoint was change from baseline in MG-ADL score at Week 26 in the combined population. Key secondary endpoints included change from baseline in QMG scores in the combined study population; change from baseline in MG-ADL score at Week 26 for the AChR+ cohort and separately the MuSK+ cohort; and change from baseline in QMG score at Week 26 for the AChR+ cohort and separately the MuSK+ cohort. Patients who entered the study taking a corticosteroid were tapered down to prednisone 5 mg a day, starting at Week 4 to Week 24. The MINT trial also includes an optional three-year open-label treatment period.
About Generalized Myasthenia Gravis (gMG)
Generalized myasthenia gravis (gMG) is a rare, chronic, B-cell-mediated autoimmune disorder that impairs neuromuscular communication and can cause muscle weakness, trouble breathing, difficulty swallowing and impaired speech and vision. 2-4
Approximately 85% of patients with myasthenia gravis have the generalized form, or gMG. 5,6
The prevalence and incidence of gMG are increasing worldwide. 6 There are between 80,000 and 100,000 patients with myasthenia gravis in the U.S. 7,8 Approximately 85% of patients with myasthenia gravis have detectable antibodies against AChR, and approximately 7% have detectable antibodies against MuSK. 9 Global prevalence is estimated at 2-36 cases per 100,000. 10 The disease is more frequently seen in young women (age 20-30) and men aged 50 years and older. 6,10
B cells are central to the pathogenesis of gMG. The disease is thought to be primarily driven by pathogenic CD19+ plasmablasts and plasma cells that target critical proteins in the neuromuscular junction. 2-4
About UPLIZNA ® (inebilizumab-cdon)
UPLIZNA is a humanized monoclonal antibody (mAb) that causes targeted and sustained depletion of key cells that contribute to underlying disease process (autoantibody-producing CD19+ B cells, including plasmablasts and some plasma cells). The precise mechanism by which UPLIZNA exerts its therapeutic effects is unknown. After two initial infusions, patients need one dose of UPLIZNA every six months.
About UPLIZNA in NMOSD
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
UPLIZNA (inebilizumab-cdon) is indicated for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adult patients who are anti-aquaporin-4 (AQP4) antibody positive.
IMPORTANT SAFETY INFORMATION
UPLIZNA is contraindicated in patients with:
- A history of life-threatening infusion reaction to UPLIZNA
- Active hepatitis B infection
- Active or untreated latent tuberculosis
WARNINGS AND PRECAUTIONS
Infusion Reactions: UPLIZNA can cause infusion reactions, which can include headache, nausea, somnolence, dyspnea, fever, myalgia, rash, or other symptoms. Infusion reactions were most common with the first infusion but were also observed during subsequent infusions. Administer pre-medication with a corticosteroid, an antihistamine, and an anti-pyretic.
Infections: The most common infections reported by UPLIZNA-treated patients in the randomized and open-label periods included urinary tract infection (20%), nasopharyngitis (13%), upper respiratory tract infection (8%), and influenza (7%). Delay UPLIZNA administration in patients with an active infection until the infection is resolved.
Increased immunosuppressive effects are possible if combining UPLIZNA with another immunosuppressive therapy.
The risk of Hepatitis B Virus (HBV) reactivation has been observed with other B-cell-depleting antibodies. Perform HBV screening in all patients before initiation of treatment with UPLIZNA. Do not administer to patients with active hepatitis.
Although no confirmed cases of Progressive Multifocal Leukoencephalopathy (PML) were identified in UPLIZNA clinical trials, JC virus infection resulting in PML has been observed in patients treated with other B-cell-depleting antibodies and other therapies that affect immune competence. At the first sign or symptom suggestive of PML, withhold UPLIZNA and perform an appropriate diagnostic evaluation.
Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating UPLIZNA.
Vaccination with live-attenuated or live vaccines is not recommended during treatment and after discontinuation, until B-cell repletion.
Reduction in Immunoglobulins: There may be a progressive and prolonged hypogammaglobulinemia or decline in the levels of total and individual immunoglobulins such as immunoglobulins G and M (IgG and IgM) with continued UPLIZNA treatment. Monitor the level of immunoglobulins at the beginning, during, and after discontinuation of treatment with UPLIZNA until B-cell repletion especially in patients with opportunistic or recurrent infections.
Fetal Risk: May cause fetal harm based on animal data. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception during treatment and for 6 months after stopping UPLIZNA.
Adverse Reactions: The most common adverse reactions (at least 10% of patients treated with UPLIZNA and greater than placebo) were urinary tract infection and arthralgia.
About Amgen
Amgen discovers, develops, manufactures and delivers innovative medicines to help millions of patients in their fight against some of the world's toughest diseases. More than 40 years ago, Amgen helped to establish the biotechnology industry and remains on the cutting-edge of innovation, using technology and human genetic data to push beyond what's known today. Amgen is advancing a broad and deep pipeline that builds on its existing portfolio of medicines to treat cancer, heart disease, osteoporosis, inflammatory diseases and rare diseases.
In 2024, Amgen was named one of the "World's Most Innovative Companies" by Fast Company and one of "America's Best Large Employers" by Forbes, among other external recognitions . Amgen is one of the 30 companies that comprise the Dow Jones Industrial Average ® , and it is also part of the Nasdaq-100 Index ® , which includes the largest and most innovative non-financial companies listed on the Nasdaq Stock Market based on market capitalization.
For more information, visit Amgen.com and follow Amgen on X , LinkedIn , Instagram , YouTube and Threads .
Amgen Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of Amgen. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including any statements on the outcome, benefits and synergies of collaborations, or potential collaborations, with any other company (including BeiGene, Ltd. or Kyowa Kirin Co., Ltd.), the performance of Otezla® (apremilast) (including anticipated Otezla sales growth and the timing of non-GAAP EPS accretion), our acquisitions of Teneobio, Inc., ChemoCentryx, Inc., or Horizon Therapeutics plc (including the prospective performance and outlook of Horizon's business, performance and opportunities, any potential strategic benefits, synergies or opportunities expected as a result of such acquisition, and any projected impacts from the Horizon acquisition on our acquisition-related expenses going forward), as well as estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes, effects of pandemics or other widespread health problems on our business, outcomes, progress, and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in the Securities and Exchange Commission reports filed by Amgen, including our most recent annual report on Form 10-K and any subsequent periodic reports on Form 10-Q and current reports on Form 8-K. Unless otherwise noted, Amgen is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future.
Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in Puerto Rico , and also depend on third parties for a portion of our manufacturing activities, and limits on supply may constrain sales of certain of our current products and product candidate development. An outbreak of disease or similar public health threat, such as COVID-19, and the public and governmental effort to mitigate against the spread of such disease, could have a significant adverse effect on the supply of materials for our manufacturing activities, the distribution of our products, the commercialization of our product candidates, and our clinical trial operations, and any such events may have a material adverse effect on our product development, product sales, business and results of operations. We rely on collaborations with third parties for the development of some of our product candidates and for the commercialization and sales of some of our commercial products. In addition, we compete with other companies with respect to many of our marketed products as well as for the discovery and development of new products. Further, some raw materials, medical devices and component parts for our products are supplied by sole third-party suppliers. Certain of our distributors, customers and payers have substantial purchasing leverage in their dealings with us. The discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to collaborate with or acquire other companies, products or technology, and to integrate the operations of companies or to support the products or technology we have acquired, may not be successful. There can be no guarantee that we will be able to realize any of the strategic benefits, synergies or opportunities arising from the Horizon acquisition, and such benefits, synergies or opportunities may take longer to realize than expected. We may not be able to successfully integrate Horizon, and such integration may take longer, be more difficult or cost more than expected. A breakdown, cyberattack or information security breach of our information technology systems could compromise the confidentiality, integrity and availability of our systems and our data. Our stock price is volatile and may be affected by a number of events. Our business and operations may be negatively affected by the failure, or perceived failure, of achieving our environmental, social and governance objectives. The effects of global climate change and related natural disasters could negatively affect our business and operations. Global economic conditions may magnify certain risks that affect our business. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase our common stock. We may not be able to access the capital and credit markets on terms that are favorable to us, or at all.
The scientific information discussed in this news release relating to new indications for our product is preliminary and investigative and is not part of the labeling approved by the U.S. Food and Drug Administration for the product. The product is not approved for the investigational use(s) discussed in this news release, and no conclusions can or should be drawn regarding the safety or effectiveness of the product for these uses.
CONTACT: Amgen, Thousand Oaks
Madison Howard , 773-636-4910 (media)
Elissa Snook , 609-251-1407 (media)
Justin Claeys , 805-313-9775 (investors)
References
- Nowak R, Phase 3 Myasthenia Gravis Inebilizumab Trial (MINT): Efficacy Data in AChR+ Generalized MG Subpopulation Through Week-52 [Late-breaking oral presentation]. To be presented at American Academy of Neurology Annual Meeting ( 5-9 April 2025 ). Available at: www.aan.com/msa/Public/Events/AbstractDetails/61470
- Yi, J. S., Guptill, J. T., Stathopoulos, P., Nowak, R. J., & O'Connor, K. C. (2018). B cells in the pathophysiology of myasthenia gravis. Muscle Nerve , 57(2):172-184.
- Willcox H. N., Newsom-Davis, J., & Calder, L. R. (1984). Cell types required for anti-acetylcholine receptor antibody synthesis by cultured thymocytes and blood lymphocytes in myasthenia gravis. Clinical and Experimental Immunology ., 58:97-106.
- Stathopoulos P., Kumar, A., Nowak, R. J., & O'Connor, K. C. (2017). Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight, 2(17):e94263.
- Lazaridis K., & Tzartos, S. J. (2020). Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics . Frontiers in Immunology, 11:212.
- Dresser L., Wlodarski, R., Rezania, K., & Soliven, B. (2021). Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J Clin Med, 10(11):2235.
- Ye et al. Frontiers in Neurology. (2024);15:1339167.
- Rodrigues E., Umeh, E., Aishwarya, Navaratnarajah, N., Cole, A., & Moy, K. (2024). Incidence and prevalence of myasthenia gravis in the United States : A claims-based analysis. Muscle Nerve, 69(2):166-171.
- Hehir, M. K., & Silvestri, N. J. (2018). Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurologic Clinics, 36:253-60.
- Bubuioc, A. M., Kudebayeva, A., Turuspekova, S., Lisnic, V., & Leone, M. A. (2021). The epidemiology of myasthenia gravis. Journal of Medicine and Life, 14(1):7-16.
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/uplizna-inebilizumab-cdon-significantly-improves-generalized-myasthenia-gravis-symptoms-in-acetylcholine-receptor-autoantibody-positive-patients-over-52-weeks-302401082.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/uplizna-inebilizumab-cdon-significantly-improves-generalized-myasthenia-gravis-symptoms-in-acetylcholine-receptor-autoantibody-positive-patients-over-52-weeks-302401082.html
SOURCE Amgen

News Provided by PR Newswire via QuoteMedia
Cizzle Brands Corporation Schedules Second Fiscal Quarter 2025 Financial Results Webcast for Thursday, March 27, 2025 at 4:30 PM ET
Following the release of Cizzle Brands' second fiscal quarter 2025 financial results, the Company's management team will be hosting a webcast to provide further insight regarding the results in the context of the Company's ongoing business activities.
Cizzle Brands Corporation (Cboe Canada: CZZL) (OTCQB: CZZLF) (Frankfurt: 8YF) ( the "Company" or "Cizzle Brands") , today announced its plans to release financial results for the second quarter of its fiscal 2025 year ended January 31, 2025 (" FQ2 2025 ") after market close on Monday, March 17, 2025. These results will be available on Cizzle Brands' profile on SEDAR+ ( http://www.sedarplus.ca/ ).
Cizzle Brands will conduct a webcast to discuss these results on the Thursday of the following week. Information regarding the webcast is provided below:
- Date and Time: Thursday, March 27, 2025 at 4:30 pm Eastern
- Scheduled Duration: Approximately one hour
- Registration Link: https://streamyard.com/watch/htXpXqNJzhUR
The webcast's registration link may not be visible through some news aggregation services. Please refer to the News section of the Cizzle Brands' website for a version of this press release with the link. Alternatively, the link may be obtained by contacting Cizzle Brands' Investor Relations team.
Cizzle Brands' Founder, Chairman, and Chief Executive Officer John Celenza commented, "As a public company with our common shares traded in three different markets, we take pride in being transparent and communicative with our growing investor base. To that end, we are pleased to be hosting this webcast following the publication of our FQ2 2025 financial results, where we will be able to provide key, plain-language insights with respect to what is behind the numbers, and how they fit into our current operations as we continue to commercialize our brands CWENCH Hydration™ and Spoken Nutrition. Our management team is excited to deliver this presentation, and we encourage all current and prospective investors in Cizzle Brands to register to attend."
About Cizzle Brands Corporation
Cizzle Brands Corporation is a sports nutrition company that is elevating the game in health and wellness. Through extensive collaboration and testing with leading athletes and trainers across several elite sports, Cizzle Brands has launched two leading product lines in the sports nutrition category: (i) CWENCH Hydration™, a better-for-you sports drink that is now carried in over 1,200 stores in Canada, the United States, and Europe; and (ii) Spoken Nutrition, a premium brand of athlete-grade nutraceuticals that carry the prestigious NSF Certified for Sport® qualification. All Cizzle Brands products are designed to help people achieve their best in both competitive sports and in living a healthy, vibrant, active lifestyle.
For more information about Cizzle Brands, please visit: https://www.cizzlebrands.com/
For more information about CWENCH Hydration™, please visit: https://www.cwenchhydration.com
For more information about the CWENCH All Canadian Games, please visit: https://www.cwenchallcanadian.com
Notice Regarding Images and Links: This press release may contain images and/or links to outside web pages, which could play an important role in providing the full context of the news update being conveyed through this press release. Some news aggregation services may remove these images and/or links at their discretion. Therefore, readers are encouraged to access SEDAR+ or the News section of the Cizzle Brands Corporation website to view this press release containing all images and/or links as originally published.
On behalf of the Board of Directors of the Company,
"John Celenza"
John Celenza, Founder, Chairman, and Chief Executive Officer
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This news release contains "forward-looking information" which may include, but is not limited to, information with respect to the activities, events or developments that the Company expects or anticipates will or may occur in the future, such as, but not limited to: new products of the Company and potential sales and distribution opportunities. Such forward-looking information is often, but not always, identified by the use of words and phrases such as "plans", "expects", "is expected", "budget", "scheduled", "estimates", "forecasts", "intends", "anticipates", or "believes" or variations (including negative variations) of such words and phrases, or state that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved. Various assumptions or factors are typically applied in drawing conclusions or making the forecasts or projections set out in forward-looking information. Those assumptions and factors are based on information currently available to the Company.
Forward looking information involves known and unknown risks, uncertainties and other risk factors which may cause the actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking information. Such risks include risks related to increased competition and current global financial conditions, access and supply risks, reliance on key personnel, operational risks, regulatory risks, financing, capitalization and liquidity risks. Although the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such information will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking information. The Company undertakes no obligation, except as otherwise required by law, to update these forward-looking statements if management's beliefs, estimates or opinions, or other factors change.
Footnotes
1 72% sequential growth of CWENCH Hydration™ 10-count units sold from Cizzle Brands' Fiscal Q1 2025 to Fiscal Q2 2025 reflects unit sales increasing from 11,943 units to 20,543 units each quarter with this account
2 64% sequential growth of CWENCH Hydration™ 315-gram units sold from Cizzle Brands' Fiscal Q1 2025 to Fiscal Q2 2025 reflects unit sales increasing from 2,730 units to 4,478 units each quarter with this account
3 39% sequential growth of CWENCH Hydration™ RTD beverage units sold during the calendar month of September 2024 compared to the calendar month of January 2025 with this account reflects unit sales increasing from 11,695 units per month to 16,280 units per month

View source version on businesswire.com: https://www.businesswire.com/news/home/20250313759799/en/
For further information, please contact:
Setti Coscarella
Head of Corporate Development
investors@cizzlebrands.com
1-844-588-2088
News Provided by Business Wire via QuoteMedia
Bird Flu Vaccine Stocks: 8 Companies Developing H5N1 Vaccines
Life science companies developing bird flu vaccines are gaining attention as the avian influenza subtype H5N1 becomes an increasing concern.
The United States is in the midst of an H5N1 bird flu outbreak that began in February 2024 and is now threatening the nation’s poultry and cattle industries. With poultry farmers across the US needing to cull their flocks if the virus is detected to prevent it spread, egg prices are shocking shoppers at the country’s grocery stores. Highly pathogenic avian influenza (HPAI) has also spread to cattle and other mammals, including cats.
Human avian influenza cases have so far been rare during this outbreak in the US, as currently the virus is only spread to humans through exposure to infected animals. As of February 27, 2025, 67 human cases have been detected in the country, and one death has been reported. However, concerns such as the possibility of mutations that could increase the chance of human-to-human transmission are stoking calls for better preparedness and access to bird flu vaccines.
In this article:
Is there a vaccine for bird flu?
There are several bird flu vaccines approved for treating avian influenza in humans, with others under development.
The Center for Disease Control and Prevention (CDC), a US federal agency under the Department of Health and Human Services, currently holds three different US Food and Drug Administration (FDA) approved vaccines in its strategic national stockpile that can be rapidly updated to address the current strain.
In the United States, the vaccines would be reserved for workers in the poultry industry if human cases escalate and could be scaled up further if needed in the case of a bird flu pandemic in humans.
Health Canada has authorized two H5N1 vaccines and laid out a framework for deciding whether to use the vaccines in a non-pandemic context, including increased human cases, human-to-human transmission and increasing severity of outcomes.
Which companies are producing vaccines for bird flu?
Some of the biggest companies in the pharmaceutical industry are either producing vaccines for bird flu or actively developing new drug candidates to fight the virus. There are also a number of large-cap and small-cap life science companies with avian influenza vaccines under development.
Below are eight bird flu vaccine stocks for investor consideration and details of their work on avian influenza. The stocks are listed by market cap based on figures retrieved from TradingView's stock screener on March 12, 2025.
1. Sanofi (NASDAQ:SNY)
Market cap: US$148.84 billion
Sanofi develops therapeutic products for diabetes and cardiovascular diseases, oncology, immunology, multiple sclerosis, rare diseases, and rare blood disorders. The French multinational pharmaceutical company is also one of the world's largest manufacturers of vaccines.
Sanofi's H5N1 vaccine became the first to be approved by the US FDA back in 2007. Today, it is one of only three US FDA-approved H5N1 vaccines held in the US national stockpile, joined by vaccines from two other pharma firms on this list, CSL Seqirus and GSK.
In October 2024, the three pharma companies were awarded a combined US$72 million by the US Administration for Strategic Preparedness and Response. The companies will prepare doses of their vaccines to be available if needed, and "manufacture additional bulk influenza antigen ... from seed stocks that are well matched to circulating strains."
2. Pfizer (NYSE:PFE)
Market cap: US$147.29 billion
Pfizer is a world-renowned research pharmaceutical company developing drugs in a wide range of areas, including oncology, inflammation and immunology, vaccines, internal medicine and rare diseases. Pfizer and BioNTech created the first FDA-approved mRNA-based COVID-19 vaccine in 2020.
Pfizer's mRNA technology could be targeted at producing an avian flu vaccine. In a May 2024 press release, the company stated that it is prepared to address an H5 group influenza pandemic, and reported that in late 2023 it had "initiated a randomized Phase 1 study to evaluate the safety, tolerability, and immunogenicity of multiple doses of nucleoside-modified mRNA (modRNA) based pandemic influenza vaccine candidate."
3. GSK (NYSE:GSK)
Market cap: US$81.76 billion
British multinational biotech company GSK has three main business divisions: pharmaceuticals, consumer healthcare and vaccines. Its vaccine Arexvy is the world’s first respiratory syncytial virus (RSV) vaccine for older adults and is approved for ages 50 and up.
GSK subsidiary ID Biomedical Corporation of Quebec produces Arepanrix, an H5N1 virus monovalent vaccine, is among the three avian flu vaccines in the US stockpile.
“GSK’s H5N1 pandemic vaccine can generate some cross-neutralizing antibodies against the current circulating strains and is recognized as an important tool in reducing illness during a possible H5N1 pandemic,” a GSK spokesperson told PharmaVoice. “The vaccine is designed to be updated with the latest circulating strain of interest, as identified by the WHO.”
In February 2025, the Public Health Agency of Canada announced that through an existing deal with GSK, it has secured an initial supply of 500,000 doses of its avian influenza vaccine.
GSK also has a mRNA-based H5N1 pre-pandemic vaccine in Phase 2 studies for adults 18 and older. GSK's mRNA candidate vaccines were previously being developed in partnership with German biopharma CureVac, another company on this list. However, the two restructured the partnership in July 2024, and GSK now has full rights to development, manufacturing and commercialization.
4. CSL (ASX:CSL,OTCQX:CMXHF)
Market cap: US$75.51 billion
Australian multinational biotechnology firm CSL is the parent company of CSL Seqirus, one of the world's largest influenza vaccine makers. CSL Seqirus has production facilities in the United States, the United Kingdom and Australia.
CSL Seqirus’ Audenz is among the three avian flu vaccines that make up US stockpiles. The company describes Audenz, which the FDA approved in 2020, as "the first-ever adjuvanted, cell-based influenza vaccine designed to protect against influenza A (H5N1) in the event of a pandemic."
CSL Seqirus has a manufacturing facility in North Carolina that was built through a public-private partnership with the US government in 2009. According to the company, the facility is the world’s largest cell-based influenza vaccine producer and its highly scalable production method means it's capable of delivering 150 million influenza vaccine doses within a six-month timeframe as part of an influenza pandemic response.
5. Moderna (NASDAQ:MRNA)
Market cap: US$13.03 billion
Moderna leads the world in the field of mRNA-based medicine from immuno-oncology to infectious diseases, as best demonstrated by its rapid deployment of effective COVID-19 vaccines. The company’s integrated manufacturing plant allows for both clinical and commercial production.
Moderna’s mRNA-based bird flu vaccine mRNA-1018 is undergoing a Phase 1/2 study targeting H5 and H7 avian influenza viruses.
In January 2025, the US Department of Health and Human Services (HHS) under the Biden Administration stated it would award Moderna US$590 million to “accelerate the development of mRNA-based pandemic influenza vaccines and enhance mRNA platform capabilities so that the U.S. is better prepared to respond to other emerging infectious diseases.” This includes its investigational avian flu vaccine.
Bloomberg reported in late February that funding is now in question as Robert F. Kennedy Jr., a long-time anti-vaccine activist, has taken the reins of the HHS under the Trump administration. Republican lawmakers in several states are also putting forth legislation to ban mRNA vaccines.
6. Novavax (NASDAQ:NVAX)
Market cap: US$1.27 billion
American vaccine developer Novavax has a pipeline of early and late-stage vaccine candidates targeting respiratory viruses and other serious infectious diseases. The biotech’s platform is based on its proprietary recombinant protein-based nanoparticle and Matrix-M adjuvant technology.
Sanofi signed a US$1.2 billion co-exclusive license in May 2024 to co-commercialize Novavax’s adjuvanted COVID-19 vaccine through much of the world.
Novavax is also conducting pre-clinical studies on a vaccine for H5N1 avian pandemic influenza using its novel approach to immunization. According to the company, "Non-human primate studies have shown (its) vaccine candidate can produce protective levels of immunity after a single dose."
7. CureVac (NASDAQ:CVAC)
Market cap: US$708.81 million
CureVac is a pioneer in developing mRNA medicines, and the first biotech company in the world “to successfully harness mRNA for medical purposes,” according to its company website. The company’s mRNA-based pipeline is based its on its proprietary RNA technology platform. It focuses on three therapeutic areas: prophylactic vaccines, cancer immunotherapies and molecular therapies.
CureVac also has an in-house GMP manufacturing facility capable of large-scale production of vaccine doses.
In 2024, CureVac, in partnership with GSK, began a Phase 1/2 study in the United States on an investigational mRNA-based bird flu vaccine for healthy younger adults aged 18 to 64 and healthy older adults aged 65 to 85 years of age. The vaccine candidate has since been fully licensed to GSK.
8. Arcturus Therapeutics (NASDAQ:ARCT)
Market cap: US$358.25 million
California-based Arcturus Therapeutics is a global commercial mRNA medicines and vaccines company. Its pipeline is focused on the development of infectious respiratory disease vaccines.
Arcturus is developing an avian flu vaccine based on its STARR self-amplifying mRNA vaccine platform technology. In 2022, the company was awarded US$63.2 million by the US HHS to support development of this vaccine for rapid pandemic influenza response. Phase 1 clinical trials for its H5N1 vaccine candidate began in January and is fully funded by the Biomedical Advanced Research and Development Authority, part of the US HHS.
Antiviral influenza stocks
Life science stocks with commercial or clinical-stage antiviral influenza medications are also worth considering for investors interested in bird flu stocks. Here are a few to get you started, listed in alphabetical order.
CoCrystal Pharma (NASDAQ:COCP)
CoCrystal Pharma is a clinical-stage biotech company with a focus on developing antiviral treatments, specifically for influenza, norovirus and COVID-19. The company’s oral influenza PB2 inhibitor CC-42344 is targeted at pandemic and seasonal influenza. Currently in Phase 2a studies, the treatment has shown in vitro activity against the avian influenza A PB2 protein.
NanoViricides (NYSEAMERICAN:NNVC)
NanoViricides is a clinical stage nanomedicine technology company. Its lead drug candidate is NV-387, a broad spectrum antiviral therapy that works by mimicking a host-side signature that viruses respond to, meaning it should be effective even as viruses mutate over time. NV-837 is developed to treat respiratory viral infections such as RSV, COVID, Long COVID, and H5N1 as well as Mpox, smallpox and measles infections. The company has successfully completed Phase 1 studies.
Roche (OTCQX:RHHBY,SWX:RO)
Switzerland-headquartered F. Hoffmann-La Roche, commonly known as Roche, is one of the world’s largest pharmaceutical companies by revenue. Along with hematology, oncology, neuroscience, and women’s health, the company also targets infectious diseases. Its drug Tamiflu is one of the leading seasonal influenza antiviral treatments, and it can be used to treat avian flu as well.
Traws Pharma (NASDAQ:TRAW)
Traws Pharma is a clinical stage company leveraging its expertise in small molecule chemistry, artificial intelligence and machine learning in the efficient development of medicines addressing respiratory viral diseases. The company's single-dose H5N1 bird flu antiviral, tivoxavir marboxil, is entering Phase 2 studies in the first half of 2025.
FAQs for bird flu vaccines
Is there a bird flu vaccine for chickens?
There are bird flu vaccines for chickens, and farmers in nations such as China, France, Egypt and Mexico use them to inoculate their flocks.
However, the avian flu vaccines for birds are not commonly used in the United States as they pose logistical challenges and create barriers to trade. In terms of trade, some US trading partners won’t purchase vaccinated chickens as the vaccine can mask an avian flu infection.
Instead, biosecurity measures such as sanitation and protective wear for workers, and culling of infected flocks are more common practices in the United States.
In response to the current bird flu outbreak, in mid-February 2025, the US Department of Agriculture conditionally approved a bird flu vaccine for chickens made by Zoetis (NYSE:ZTS), the world's largest producer of medicine and vaccinations for pets and livestock.Is there a bird flu vaccine for cattle?
There are bird flu vaccines for cattle under development. For example, Medgene, a privately held animal health company based in South Dakota, is developing an H5N1 vaccine for cattle that as of late February 2025 is waiting on imminent conditional approval from the US Department of Agriculture. The company has signed a distribution agreement with global animal health company Elanco Animal Health (NYSE:ELAN) for the vaccine.
Is there a bird flu vaccine for cats and dogs?
While both animals can catch avian flu, there are no commercial bird flu vaccines are currently available for cats and dogs. Cats are at higher risk of contracting HPAI bird flu than dogs, but owners of both should take precautionary measures.
The American Veterinary Medical Association advises cats should be kept indoors. Pet owners should keep outdoor pets, including backyard chicken flocks, away from the wild birds, poultry and cattle.
Additionally, pet owners must avoid feeding pets raw meat or poultry and unpasteurized milk, and prevent pets from eating dead birds or other animals.
Don’t forget to follow @INN_LifeScience for real-time updates!
Securities Disclosure: I, Melissa Pistilli, hold no direct investment interest in any company mentioned in this article.
Latest News

Cleo Diagnostics Investor Kit
- Corporate info
- Insights
- Growth strategies
- Upcoming projects
GET YOUR FREE INVESTOR KIT
Latest Press Releases
Related News
TOP STOCKS
Investing News Network websites or approved third-party tools use cookies. Please refer to the cookie policy for collected data, privacy and GDPR compliance. By continuing to browse the site, you agree to our use of cookies.







